د ۲۰۲۲ کال په می میاشت کې، د شینزین JYMed ټیکنالوژۍ شرکت، لمیټډ (له دې وروسته د JYMed پیپټایډ په نوم یادیږي) د متحده ایالاتو د خوړو او درملو ادارې (FDA) ته د سیماګلوټایډ API د راجسټریشن لپاره غوښتنلیک وسپاره (د DMF راجسټریشن شمیره: 036009)، دا د بشپړتیا بیاکتنه تیره کړې، او اوسنی حالت یې "A" دی. JYMed پیپټایډ په چین کې د سیماګلوټایډ API جوړونکو لومړۍ ډله شوه چې د متحده ایالاتو FDA بیاکتنه یې تیره کړه.
د ۲۰۲۳ کال د فبروري په ۱۶مه، د دولتي درملو ادارې د درملو ارزونې مرکز رسمي ویب پاڼې اعلان وکړ چې د سیماګلوټایډ API [د ثبت شمیره: Y20230000037] د هوبي JXBio Co., Ltd لخوا ثبت او اعلان شوی، چې د JYMed پیپټایډ فرعي شرکت دی، منل شوی دی. JYMed پیپټایډ د لومړنیو خامو موادو درملو تولیدونکو څخه یو شو چې د دې محصول لپاره د بازار موندنې غوښتنلیک یې په چین کې منل شوی دی.
د سیماګلوټایډ په اړه
سیماګلوټایډ د GLP-1 ریسیپټر اګونیسټ دی چې د نوو نورډیسک (نوو نورډیسک) لخوا رامینځته شوی. دا درمل کولی شي د انسولین د خپریدو لپاره د پانقراس β حجرو هڅولو سره د ګلوکوز میتابولیزم زیات کړي، او د پانقراس α حجرو څخه د ګلوکاګون سرایت مخه ونیسي ترڅو روژه او د خوړو وروسته د وینې شکر کم کړي. سربیره پردې، دا د اشتها کمولو او په معدې کې د هضم ورو کولو سره د خواړو مصرف کموي، کوم چې په نهایت کې د بدن غوړ کموي او د وزن کمولو کې مرسته کوي.
۱. اساسي معلومات
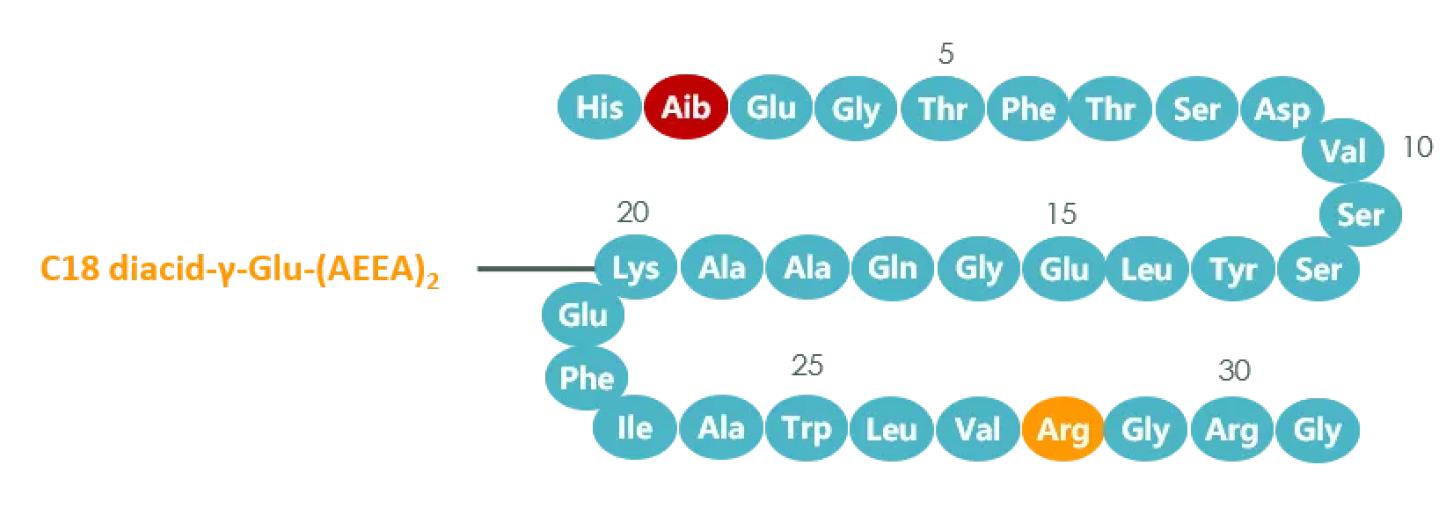
د جوړښت له نظره، د لیراګلوټایډ په پرتله، د سیماګلوټایډ ترټولو لوی بدلون دا دی چې د لیسین د اړخ زنځیر ته دوه AEEAs اضافه شوي، او پالمیټیک اسید د اوکټاډیکانیډیویک اسید لخوا بدل شوی. الانین د ایب لخوا بدل شو، کوم چې د سیماګلوټایډ نیم ژوند خورا اوږد کړ.
د سیماګلوټایډ جوړښت شکل
2. نښې نښانې
۱) سیماګلوټایډ کولی شي د T2D ناروغانو کې د زړه او رګونو د ناروغیو خطر کم کړي.
۲) سیماګلوټایډ د انسولین سرایت هڅولو او د ګلوکاګون سرایت کمولو سره د وینې شکر کموي. کله چې د وینې شکر لوړ وي، د انسولین سرایت هڅول کیږي او د ګلوکاګون سرایت مخنیوی کیږي.
۳) د نوو نورډیسک PIONEER کلینیکي آزموینې ښودلې چې د سیماګلوټایډ ۱ ملی ګرامه، ۰.۵ ملی ګرامه شفاهي اداره د ټرولیسیټي (ډولاګلوټایډ) ۱.۵ ملی ګرامه، ۰.۷۵ ملی ګرامه په پرتله ښه هایپوګلیسیمیک او د وزن کمولو اغیزې لري.
۳) د خولې له لارې سیماګلوټایډ د نوو نورډیسک د ټرمپ کارت دی. په ورځ کې یو ځل د خولې له لارې ورکول کولی شي د انجیکشن له امله رامینځته شوي تکلیف او رواني شکنجې څخه خلاص شي، او دا د لیراګلوټایډ (په اونۍ کې یو ځل انجیکشن) څخه غوره دی. د عام درملو لکه ایمپاګلیفلوزین (SGLT-2) او سیټاګلیپټین (DPP-4) هایپوګلیسیمیک او د وزن کمولو اغیزې ناروغانو او ډاکټرانو ته خورا زړه راښکونکي دي. د انجیکشن فورمولونو سره پرتله کول، شفاهي فورمولونه به د سیماګلوټایډ د کلینیکي غوښتنلیک اسانتیا خورا ښه کړي.
۳. لنډیز
دا په دقیقه توګه د هایپوګلیسیمیک، وزن کمولو، خوندیتوب او د زړه او رګونو په ګټو کې د غوره فعالیت له امله ده چې سیماګلوټایډ د لوی بازار احتمال سره د پدیدې په کچه "نوی ستوری" ګرځیدلی دی.
د پوسټ وخت: فبروري-۱۷-۲۰۲۳