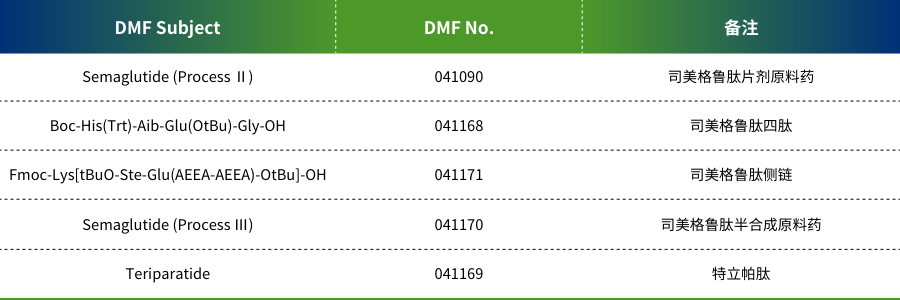
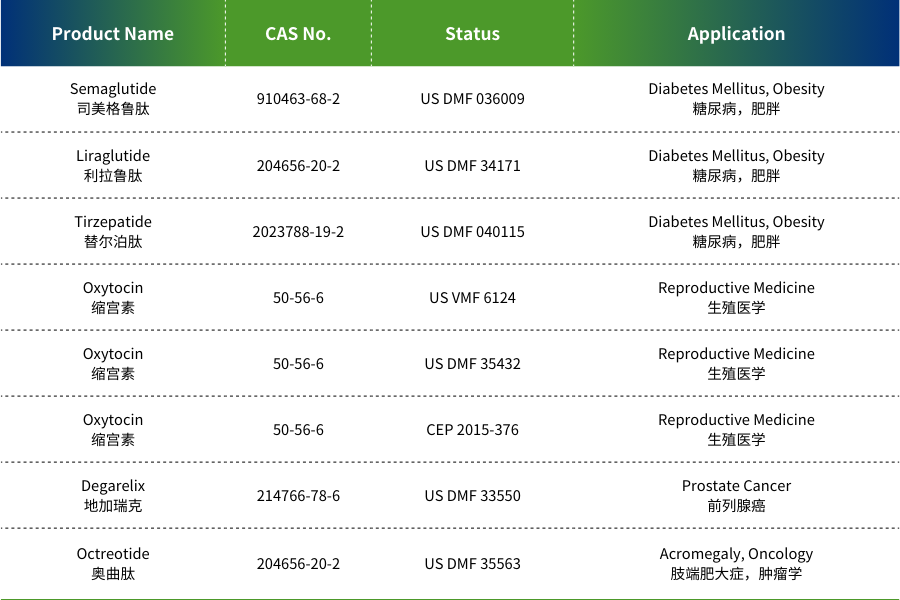
N'oge na-adịbeghị anya, Shenzhen JYMed Technology Co., Ltd. (nke a na-akpọ "JYMed") emechaala akwụkwọ ndekọ Drug Master File (DMF) maka ngwaahịa ise ọzọ na US Food and Drug Administration (FDA), na-agbasawanye Pọtụfoliyo ngwaahịa ya.
Banyere JYMED
JYMed bụ a elu-tech biopharmaceutical ụlọ ọrụ pụrụ iche na onwe nnyocha, mmepe, mmepụta, na ahịa nke peptide dabeere na ngwaahịa, nakwa dị ka nkwekọrịta mmepe na n'ichepụta nzukọ (CDMO). Ụlọ ọrụ ahụ na-agba mbọ ịnye API peptide dị elu yana ngwọta ahaziri maka ndị ahịa ụwa. Pọtụfoliyo ngwaahịa ya gụnyere ọtụtụ peptide API, yana ngwaahịa ndị dị ka Semaglutide na Terlipressin emechaala akwụkwọ US FDA DMF.
Ndị enyemaka ya, Hubei JXBio Pharmaceutical Co., Ltd., na-arụ ọrụ ahịrị mmepụta peptide API ọgbara ọhụrụ na-agbaso ụkpụrụ cGMP nke US FDA, European EMA, na NMPA nke China setịpụrụ. Ụlọ ọrụ ahụ na-agụnye ahịrị mmepụta 10 nnukwu na pilot ma guzobe usoro nlekọta ahụike siri ike (QMS) na usoro nlekọta ahụike na nchekwa gburugburu ebe obibi (EHS). Ndị a na-achọpụta na usoro dum, site na R&D ruo mmepụta, na-ezute ụkpụrụ mba ụwa kachasị elu. Ụlọ ọrụ ahụ agafeela nyocha nnabata GMP site na US FDA na NMPA nke China na ndị ụlọ ọrụ na-ahụ maka ọgwụ zuru ụwa ọnụ nabatara ya maka ịdị mma njikwa EHS ya, na-egosipụta nraranye ya pụtara ìhè n'ịdị mma, nchekwa, na ọrụ gburugburu ebe obibi.
Mpaghara azụmahịa isi: Ndebanye aha na nnabata peptide API nke ụlọ na nke mba ụwa, peptides ọgwụgwọ anụmanụ na ịchọ mma, ọrụ peptide ahaziri iche, gụnyere CRO, CMO, na ngwọta OEM, conjugates peptide-drug conjugates (PDCs), gụnyere peptide-radionuclide, peptide-obere molecule, peptide-protein.
Isi ngwaahịa
Maka nkọwa ndị ọzọ gbasara ngwaahịa anyị, biko kpọtụrụ anyị.
API zuru ụwa ọnụ na ajụjụ ịchọ mma: Tel No.: +86-15013529272;
Ndebanye aha API & Ọrụ CDMO (ahịa USA EU): +86-15818682250
Email:jymed@jymedtech.com
Adreesị: Ụlọ elu 8 & 9, Ụlọ 1, Shenzhen Biomedical Innovation Industrial Park, 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Oge nzipu: Mar-25-2025