Shenzhen JYMed Technology Co., Ltd minangka perusahaan teknologi tinggi sing melu riset lan pangembangan, manufaktur lan komersialisasi produk adhedhasar peptida kalebu peptida bahan farmasi aktif, peptida kosmetik, lan peptida khusus uga pangembangan obat peptida anyar. JYMed duwe rong anak perusahaan: Shenzhen JYBio Pharmaceutical Co., Ltd lan Hubei JY Pharmaceutical Co., Ltd, sing lagi dibangun.
【R & D tengah】
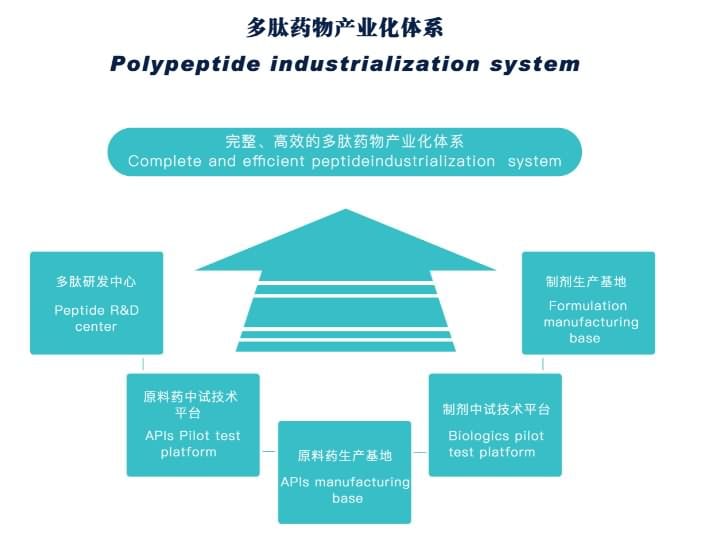
JYMed kang R & D tengah, dumunung ing Shenzhen, wis nyiyapake kanggo riset lan pangembangan bahan kimia tamba anyar, API peptida lan produk cocog. tengah dilengkapi karo modern peptida synthesizer, gedhe-kapasitas sistem dimurnèkaké preparative, lan instrumen analitis lengkap kalebu MS, HPLC, GC, UV, IC etc. Pusat R & D uga menehi support teknologi kanggo loro proses ditemokaké tamba lan Manufaktur anyar nransfer.

【Produksi Base】

JYMed wis papat garis produksi peptida API ing Nanjing sesuai karo FDA cGMP selaras, lan loro dosis garis produksi biologi rampung ing Shenzhen sing bisa nyedhiyani perangan komersial injectables peptida injectables kapasitas cilik lan produk wêdakakêna beku-pepe ing pedoman cGMP. Kajaba iku, kita mbangun nembelas garis produksi kanggo peptida API ing Xianning, provinsi Hubei, China, kang bakal dadi salah siji saka paling gedhe basa Manufaktur peptida API ing China.
JYMed has complete and efficient peptide industrialization system, can provide Comprehensive peptide service, including CRO/CMO/CDMO/OEM and Regulatory affairs support. To be Your reliable, independent and reactive supplier for your peptides!
【Key Figures of JYMed】
● 315 karyawan negoro
● 2 RD Laboratorium (Pingshan, Sky)
● 3 fasilitas Manufaktur
● API, Nanjing Lishui, Jiangsu, 4 garis produksi ing selaras karo FDA
● API, Xian'ning, Hubei, 16 garis produksi ing selaras karo FDA (ing construction)
● produk Companies, 2 garis dosis ing Shenzhen Pingshan, Guangdong
● 7 produk diajukake anda kanggo CFDA, 5 produk ing key
● 1 produk IND diajukake CFDA
● 1 produk diajukake VMF (US-FDA) lan dijupuk certificate CEP


【 Factory pictures】









JYMed offers high qualified peptide APIs, cosmetic peptide and CRO CMO service from research grade to cGMP grade with stringent quality control helping you in selecting the right protection for your application.







