Shenzhen JYMed Technologia Co.,Ltd est summus technicus inceptio in investigationibus et evolutione versatus, fabricandis et mercatura peptidum substructio productorum incluso peptides medicamentis pharmaceuticae, cosmeticae peptides, et consuetudo peptidum necnon nova evolutionis medicamentorum peptidum. JYMed duo subsidiaria omnino possident: Shenzhen JYBio pharmaceutica Co, Ltd et Hubei JY Pharmaceutical Co, Ltd, quod sub constructione est.
R & D centrum】 【
JYMed centrum in R & D, sita in Shenzhen, extruxerat esse ad novum medicamento investigationis et progressionem de substantiis, quod pertinet precursor APIs products. In medio instructa modern precursor synthesizer, magna-facultatem praeparativum aliquod minus ratio, et comprehensive analytical instruments inter MS: silicium, GC, UV, IC et cetera ad R & D centrum etiam providet technology subsidium tam novum medicamento inventionis et manufacturing processus transire.

Productio】 【Base

JYMed habet quatuor lineae peptide API productio in Nanjing cGMP obsequio FDA sicut et duas lineas in Shenzhen productio rite celebrata consectetuer biologicis potest providere batches de commercial facultatem parva Mammalia injectables injectables et Frigidus-arida in pulveris products cGMP guidelines. Et etiam, quae ad aedificationem sedecim productio lineae peptide API In Xianning, Hubei provinciae, Sinis, quod sit basis unius maximus precursor API manufacturing in Sinis.
JYMed has complete and efficient peptide industrialization system, can provide Comprehensive peptide service, including CRO/CMO/CDMO/OEM and Regulatory affairs support. To be Your reliable, independent and reactive supplier for your peptides!
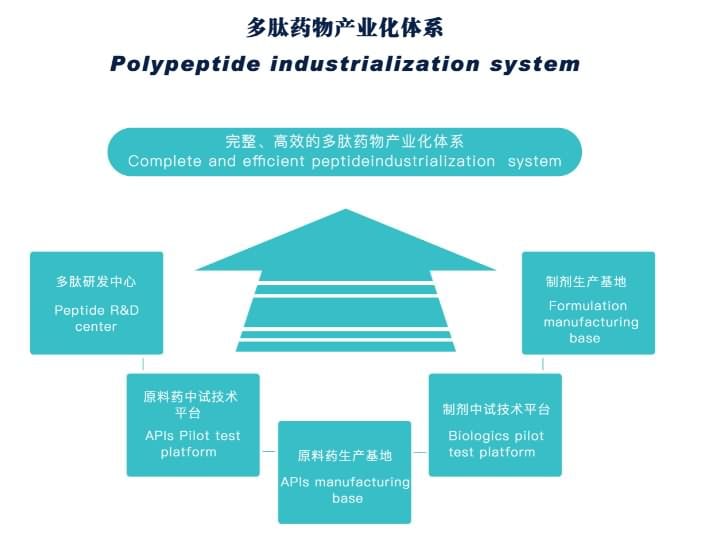
【Key Figures of JYMed】
CCCXV employees nationwide ●
● laboratorios demonstra ST II (Pingshan, Nanshan)
● III vestibulum facilities
● APIs, Nanjing Lishui, Jiangsu, IV lineae productio in obsequio cum FDA
● APIs, Xian'ning, Hubei, XVI lineas productio in obsequio cum FDA (under construction)
● compleas products, II mg per lineas Pingshan Shenzhen, Guangdong
● summitto Aurelius Clemens VII products ad CFDA, V products sub interpositione
● I uber ut summitto MEX CFDA
● I productum summitto VMF (US FDA) et certificatorium adeptus CEP


【 Factory pictures】









JYMed offers high qualified peptide APIs, cosmetic peptide and CRO CMO service from research grade to cGMP grade with stringent quality control helping you in selecting the right protection for your application.







