-
New Regulation Bulletin
1. New FDA Registration Regulations for U.S. Cosmetics Cosmetics Without FDA Registration Will Be Banned from Sale.According to the Modernization of Cosmetics Regulation Act of 2022, signed by President Biden on December 29, 2022, all cosmetics exported to the United States must be FDA-registere...Read more -
Spring Festival Holiday Notice
Please kindly be informed that our office will be closed from Feb. 4 to Feb.18 due to the Spring Festival. Any orders will be accepted but will not be processed until Feb.19, the first business day after the Spring Festival. Sorry for any inconvenience caused.Read more -

2023 The 88th API CHINA
Information about JYMed at 2023 API CHINA 【on-site】 Under the leadership of Vice General Manager Zhi Qin, Shenzhen JYMed Technology Co.,Ltd. (hereinafter referred to as JYMed)participated in this grand exhibition. JYMed showcased advantageous products Semaglutide, Liraglutide, Tirzepatide, O...Read more -
Welcome to meet us at API exhibition at Qindao China JYMed stock: N4K32
Read more -

JYMed got together with you at PCHi
After two years of expectation, the 2023 China International Cosmetics Personal and Home Care Raw Materials Exhibition (PCHi) had been held in the Guangzhou Canton Fair Complex on February 15-17, 2023. PCHi is an international trade show serving the global cosmetics, per...Read more -

Semeglutide API of Shenzhen JYMed accepted by the first batch of domestic NMPA and Registered in US FDA (DMF No. 036009) with status “A”.
In May 2022, Shenzhen JYMed Technology Co., Ltd. (hereinafter referred to as JYMed peptide) submitted an application for the registration of semaglutide API to the US Food and Drug Administration (FDA) (DMF registration number: 036009), It has passed the integrity review, and the current status i...Read more -
Copper Peptides: Skin and Hair Care Benefits and How to Use Them
We include products that we think our readers will find useful. We may earn a small commission if you make a purchase through a link on this page. This is our process. Peptides are naturally occurring amino acids that help produce collagen and elastin, the two connective tissues responsible for s...Read more -
Ozempic (Semaglutide) Injection: Uses, Side Effects, Dosage
Erica Prouty, PharmD, is a professional pharmacist assisting patients with medication and pharmacy services in North Adams, Massachusetts. In non-human animal studies, semaglutide has been shown to cause C-cell thyroid tumors in rodents. However, it is unclear whether this risk extends to humans....Read more -
Derivatization of fatty acids in the discovery of peptide and protein drugs
Thank you for visiting Nature.com. The browser version you are using has limited CSS support. For the best experience, we recommend that you use an updated browser (or disable Compatibility Mode in Internet Explorer). In the meantime, to ensure continued support, we will render the site without s...Read more -

Class I innovative drug of JYMed have made significant progress, Laipushutai is expected to become the first line of UC drugs.
On June 29, 2017, the development of Laipushutai, the class I innovative medicine with the cooperative development of JYMed and Guangzhou Linkhealth Medical Technology Co., Ltd., has made significant progress. The drug’s IND declaration has been accepted by the CFDA. JYMed and Guangzhou Lin...Read more -
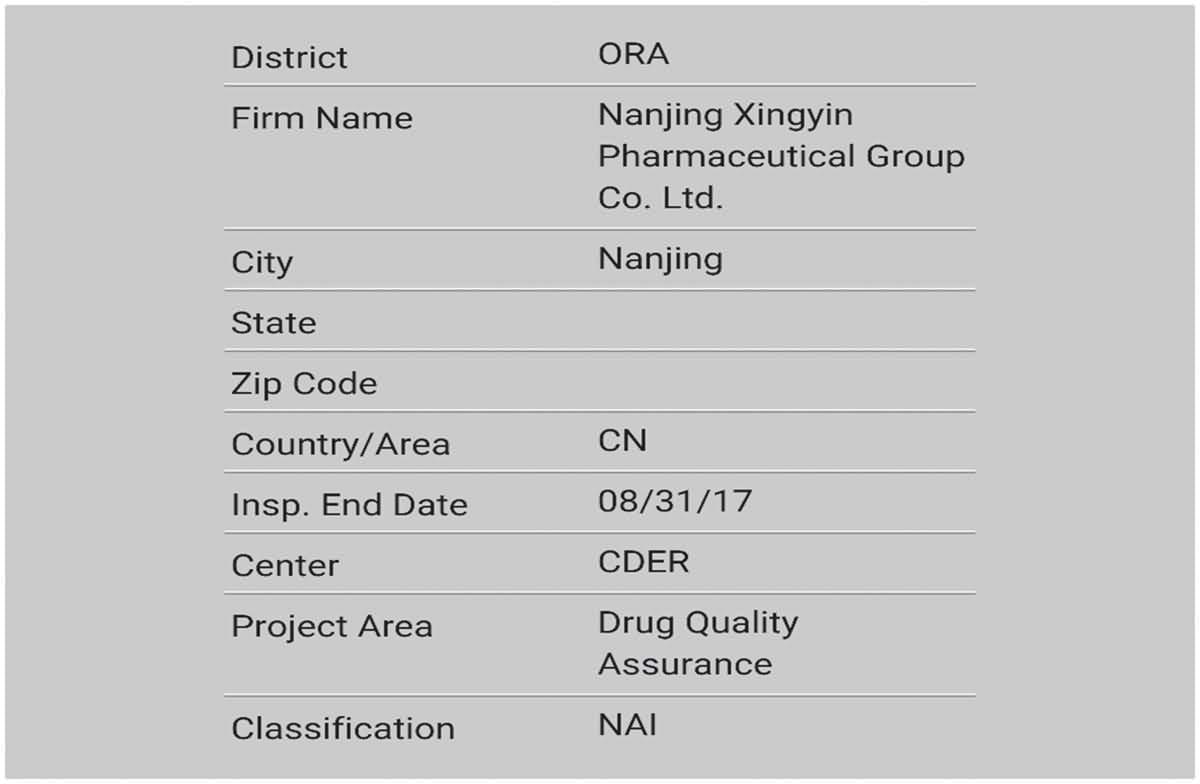
News & EventsThe Peptide Products Division in JYMed has flawlessly passed on-site inspection from FDA.
Warmly congratulate our Polypeptide Products Division for successfully passing the US FDA on-site inspection with “zero defects”! Passing the FDA on-site inspection with “zero defects” is a major event in our cGMP development history. It not only means that our API has obt...Read more
- E-mail: jymed@jymedtech.com
- Phone: +86 0755-26612112







